Learning Outcomes
By the end of this lesson, students should be able to:
i. Explain the mechanisms of acid- and base-catalyzed nucleophilic addition reactions of aldehydes and ketones.
ii. Identify the factors affecting the rate and regioselectivity of these reactions.
iii. Predict the products of acid- and base-catalyzed nucleophilic addition reactions.
iv. Provide examples of applications of these reactions in organic synthesis.
Introduction
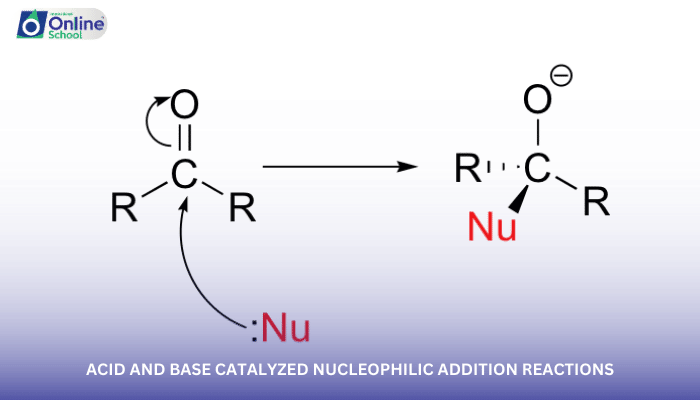
Aldehydes and ketones are carbonyl compounds that undergo nucleophilic addition reactions due to the electrophilic nature of their carbonyl carbon. These reactions can be catalyzed by acids or bases, leading to different products and reaction rates.
i. Acid-Catalyzed Nucleophilic Addition Reactions
In acid-catalyzed nucleophilic addition reactions, the acid protonates the carbonyl oxygen, making the carbonyl carbon more electrophilic and susceptible to attack by the nucleophile. The general mechanism for acid-catalyzed nucleophilic addition is shown below:
RCO2H + Nucleophile → RCO2H·Nucleophile Complex → Adduct + H2O
The rate of acid-catalyzed nucleophilic addition reactions depends on the strength of the acid, the nucleophilicity of the nucleophile, and the steric hindrance around the carbonyl carbon.
ii. Base-Catalyzed Nucleophilic Addition Reactions
In base-catalyzed nucleophilic addition reactions, the base deprotonates the α-hydrogen adjacent to the carbonyl group, generating an enolate ion. The enolate ion is a stronger nucleophile than the neutral carbonyl compound and readily reacts with electrophiles. The general mechanism for base-catalyzed nucleophilic addition is shown below:
RCOCH2R' + Base → RCOCH2R'⁻ + BaseH+
RCOCH2R'⁻ + Electrophile → Adduct
The rate of base-catalyzed nucleophilic addition reactions depends on the strength of the base, the stability of the enolate ion, and the reactivity of the electrophile.
iii. Regioselectivity of Nucleophilic Addition Reactions
The regioselectivity of nucleophilic addition reactions refers to the preference for the nucleophile to attack the carbonyl carbon from one side over the other. Acid-catalyzed nucleophilic addition reactions are typically regioselective due to steric hindrance, with the nucleophile attacking the less hindered side of the carbonyl carbon. Base-catalyzed nucleophilic addition reactions, on the other hand, are less regioselective due to the formation of a delocalized enolate ion.
iv. Applications of Nucleophilic Addition Reactions
Nucleophilic addition reactions are widely used in organic synthesis for the preparation of various compounds. Some examples include:
Hydration of Aldehydes and Ketones: Aldehydes and ketones react with water in the presence of an acid catalyst to form geminal diols.
Addition of Alcohols: Aldehydes and ketones react with alcohols to form hemiacetals or acetals, depending on the reaction conditions.
Addition of Hydrogen Cyanide: Aldehydes and ketones react with hydrogen cyanide (HCN) to form cyanohydrins.
Grignard Reactions: Aldehydes and ketones react with Grignard reagents to form alcohols.
Wittig Reactions: Aldehydes and ketones react with Wittig reagents to form alkenes.
Acid- and base-catalyzed nucleophilic addition reactions are fundamental reactions in organic chemistry, providing a versatile approach for the synthesis of various compounds. Understanding the mechanisms and regioselectivity of these reactions is essential for predicting their outcomes and designing synthetic routes.